BMT CTN 1503 Trial

What is a clinical trial?
Clinical trials are research studies that help determine if a treatment or device is safe and beneficial for patients. Clinical trials produce the best information and evidence for health care decision making.
About the BMT(Blood and Marrow Transplant Clinical Trials Network STRIDE 2) CTN 1503 Trial
Over time, as patients with SCD age, they may experience complications from their disease caused by organ damage from SCD. There is also an associated risk of premature death for adults with SCD.
BMT CTN 1503 (STRIDE 2) is a clinical trial to find out if bone marrow transplantation is better than standard of care in improving survival and decreasing sickle cell-related complications for young adults with severe SCD. To better understand which treatment option is most effective, we need a side-by-side comparison of bone marrow transplant and standard of care for SCD. BMT CTN 1503 (STRIDE 2) is the first trial to compare these treatment options for SCD. Currently, we do not know if bone marrow transplants is better than the standard of care for treating SCD in adults. However, bone marrow transplant in children 16 years old and younger have shown promising outcomes.
In the BMT CTN 1503 (STRIDE 2) study, we will learn more about the results of bone marrow transplant in comparison to the standard of care in young adult and adult patients with SCD. The National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) is funding the BMT CTN 1503 (STRIDE 2) trial. The trial is currently being conducted in 46 centers across the United States and in Canada and will enroll a total of 200 patients. Participation in this trial is entirely voluntary.
What is the study process?
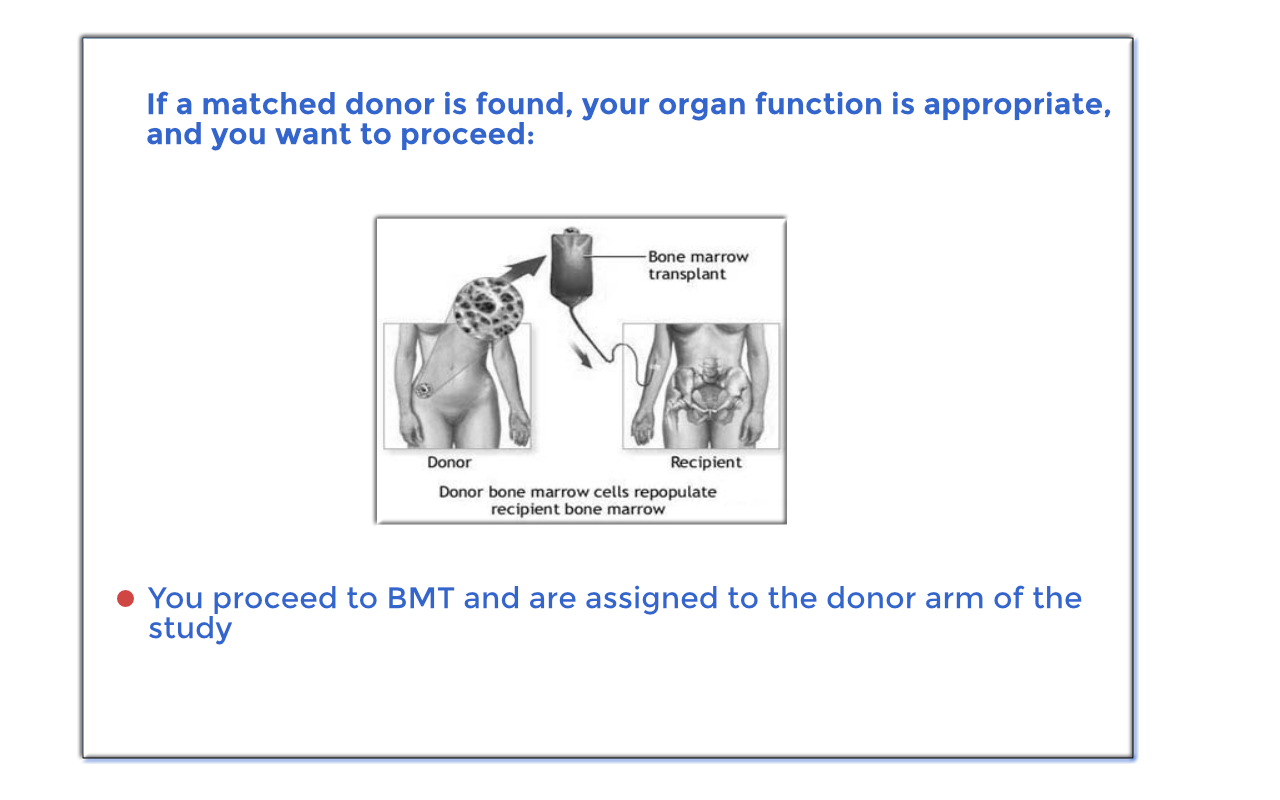
Interested participants will be screened for eligibility. If you are eligible for the study, you will be tissue typed (HLA-typed) and will undergo a donor search to determine if a suitable related or unrelated donor is available. If you have a donor match, you will be assigned to the donor arm of the study and will undergo a bone marrow transplant. If you do not have a suitable donor match, then you will be assigned to the no donor arm of the study and will continue with your usual standard of care treatment.
Learn More About the Study Process

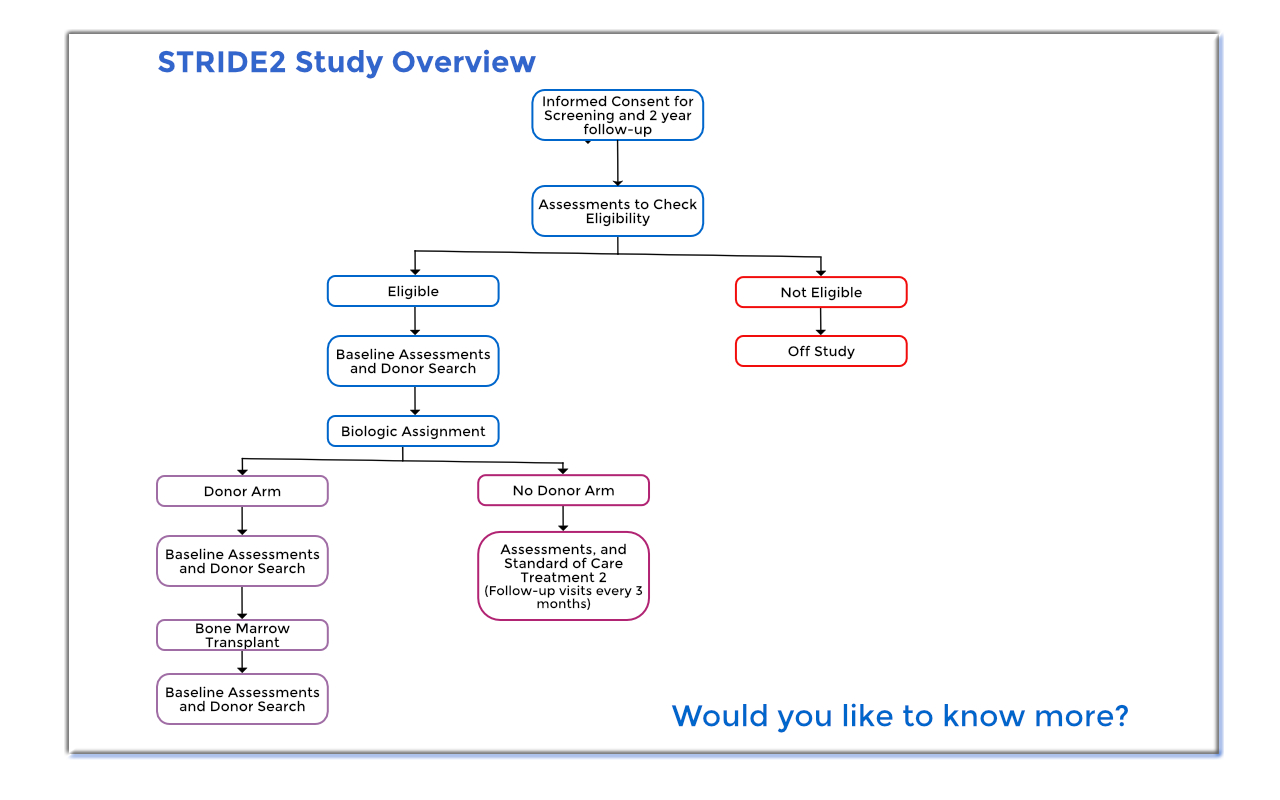







How long will I be in the study?
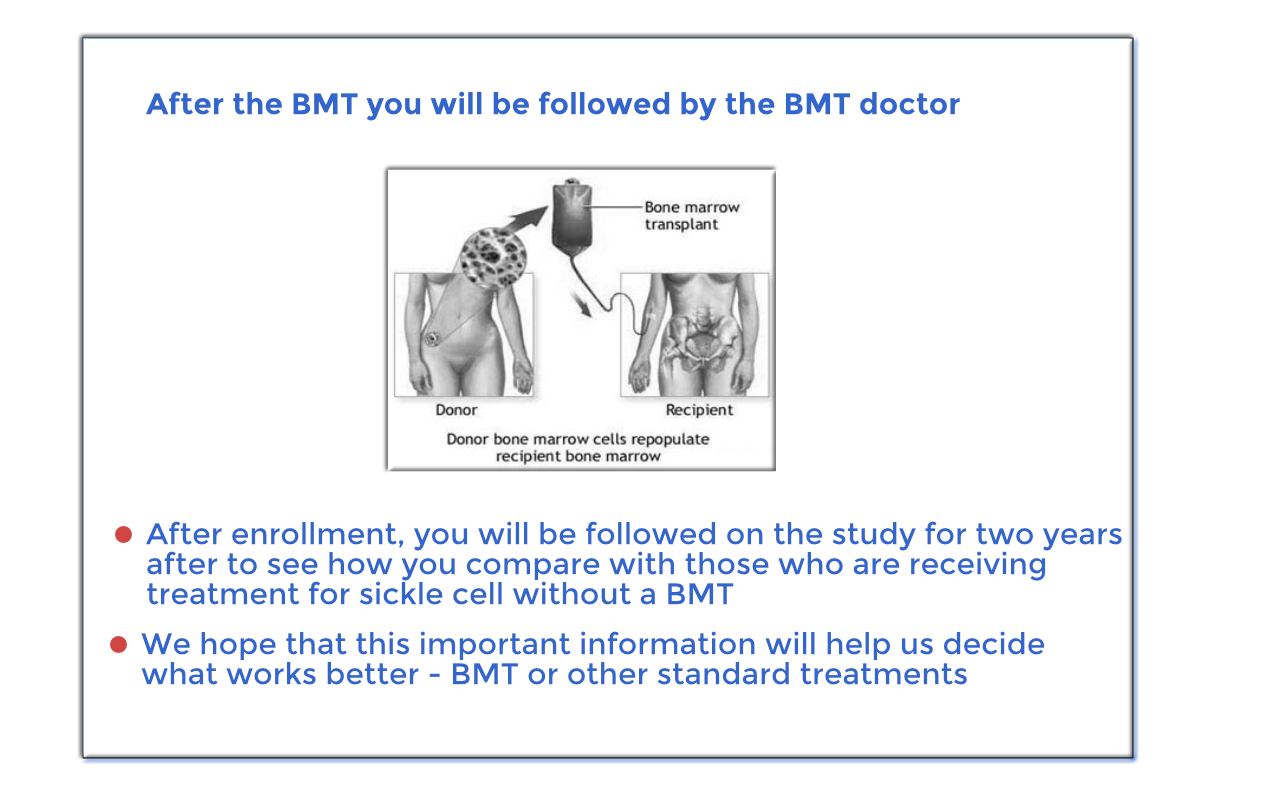
You will be in the BMT CTN 1503 (STRIDE 2) study for two years.
If you are in the no donor arm, you will have follow-up visits every three months. If you are in the donor arm and receive a transplant you will be followed by your doctor as frequently as needed.
What are the possible benefits to being in this study?
You may or may not benefit from taking part in this study. If you do not have a donor, we will collect SCD-related information from you, which will allow us to directly compare the health outcomes for patients who receive a bone marrow transplant to those patients who receive regular/routine care. If you have a donor and the bone marrow transplant is successful, you may benefit by not having any further symptoms and complications of severe SCD.
However, at this time, we do not know which treatment option has better outcomes for adolescent and adult patients with SCD. Information obtained from your participation in this study will help doctors treat patients with severe SCD in the future.
What are the possible risks of being in this study?
If you are assigned to the donor arm and proceed with a bone marrow transplant, there are risks involved in the process which include:
- Rejection of the transplant if your body does not accept the new cells
- Infection
- Infertility
- Stroke or seizure
- Graft-versus-host-disease (GVHD)- when the donor's cells attack your body
- Death
- Unknown risks
- Questionnaire risks
- Blood draw risks
- Nuclear medicine risks (GFR, MUGA)
A bone marrow transplant can be hard on patients and their families because it is such a long process.
A doctor will review all of the risks involved in participating in this study with you at the participating site during your consultation.
If you are assigned to the no donor arm, you will receive routine care prescribed by your SCD physician. Treatments received on the no donor arm are both standard of care and research tests. We will collect information about blood tests and other tests you have had when you enroll and again 2 years later. Risks include:
- Blood draw risks
- Questionnaire risks
- Nuclear medicine risks
- Other risks
What do I have to do to be in the study?
If you are interested in participating in the study, you can find a study site near you by visiting ClinicalTrials.gov or select "Find a participating site" from the "Study Information" menu above. There you will find a list of all participating sites in addition to contact information for each site. Reach out to the site you are interested in and a clinical research coordinator will get back to you as soon as possible. The coordinator will be able to address any questions or concerns you may have and can put you in touch with a transplant doctor for a consultation. You can also speak to your hematologist about your interest in participating in this trial. Lastly, you can contact a clinical research coordinator at Children’s Healthcare of Atlanta by sending an email to 1503STRIDE2_CCC@CHOA.ORG. directly. Children’s Healthcare of Atlanta is the clinical coordinating center for the trial and can help you find a participating site.
At the participating site, a doctor will explain the study and answer any questions you may have. After answering all of your questions, you will be asked to review and sign a consent/ assent form if you are interested in enrolling in the study.
Inclusion Criteria
This study is open to males and females between the age of 15 and 40 with severe SCD who have had one or more serious problems that include:
- Stroke or other serious brain complication
- 2 episodes of acute chest syndrome (ACS) within the last 2 years
- 6 or more severe pain crises in the last two years
- Receiving 8 or more blood transfusions per year to prevent SCD-related health problems
- An echocardiographic finding of a heart problem (TRJ ≥ 2.7 m/sec)
- Chronic pain on a majority of days per month for ≥ 6 months
Exclusion Criteria
Participation is not allowed for patients who:
- Have cirrhosis of the liver (a very serious condition of liver failure)
- Have or have had a very serious infection in the past six weeks
- Have an HIV infection
- Have already received a bone marrow transplant (HCT/BMT)
- Are currently pregnant or breast feeding